VERY IMPORTANT FACT ABOUT GcMAF TREATMENT
There are different generations in GcMAF therapy. The first generation GcMAF was developed over 20 years ago. Due to limited testing and clinical uses, the efficacy is unknown.
We have developed the 2nd and 3rd Generation GcMAF with a new patented production process. Therefore, our GcMAF is very unique. Please note that our 2nd Generation GcMAF should not be colourless liquid, as it is produced from serum.
Our 3rd Generation GcMAF products are classified as a food product in Japan, and they are available in forms of Capsules, Sprays and Lollies.
Our 3rd Generation GcMAF products are not produced from human serum, but bovine colostrum and cheese extract.
Oral Colostrum MAF capsule is produced from bovine colostrum. Colostrum is very similar to serum – very rich in protein, IgG, IgA and IgM. Now they are available in capsules, sprays and lollies. MAF Sprays and M-Lollies are produces from Cheese extract. All can be administered orally and topically.
Comparison of 1st and 2nd Generation GcMAF

First Generation GcMAF
- Developed by Dr Yamamoto in 1991
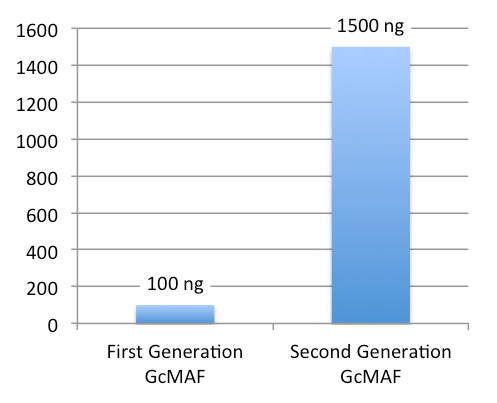
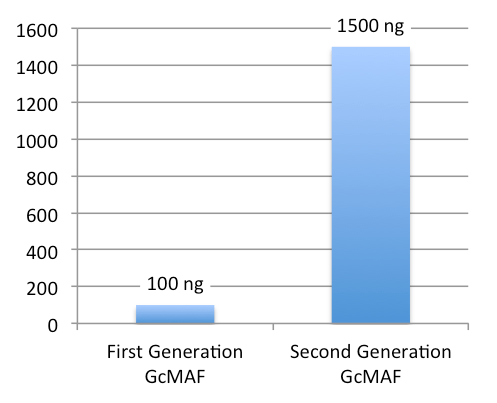
- Low concentration (100 ng/0.25 ml, 1 dose)
- Low stability at room temperature and when refrigerated
- Gc protein isolated from serum using 25-(OH) Vitamin D3 Affinity Column, making the GcMAF unstable
- Sterilized using 0.22 micron sterile filtration system
- Tokushima University conducts experiments with 1st Generation and 2nd Generation GcMAF
Second Generation GcMAF
- 2nd Generation High Dose GcMAF Developed by the University of Tokushima and Saisei Mirai in 2011
- New patented production process
- High concentration (1500 ng/0.5 ml, 1 dose)
- Very high macrophage activating activity
- Much higher stability even at room temperature *
- Demonstrated in vitro to increase the rate of maturation of Dendritic Cells (DCs).
- Sterilized using 0.22 micron sterile filtration system
Third Generation GcMAF (Capsules, Sprays and Lollies)
- Developed by Saisei Mirai and Tokushima University in 2014
- Registered as a food product in Japan
Oral Colostrum MAF Capsules
- MAF produced from bovine colostrum
- 1 mg capsule has equivalent activity to 100ng purified GcMAF
- Enteric acid-resistant capsule for oral administration, powder for sublingual
- Target Payer‘s Patches/Gut
MAF spray
- MAF produced from Cheese extract
- 1 dose (2 sprays) has equivalent activity to 100ng purified GcMAF
- Spray MAF is designed for oral and skin application.
M-Lollies
- MAF produced from Cheese extract
- 1 mg capsule has equivalent activity to 100ng purified GcMAF
- In the form of candies for even easier every day consumption.